Physician inpatient handoffs—Patient and physician outcomes: A systematic review
Joshua Allen-Dicker and Matthew Kerwin are joint first authors.
Abstract
Background
Prior reviews have shown that interventions to improve inpatient handoffs are inconsistently associated with improvement in patient outcomes. This systematic review examines the effectiveness of inpatient handoff interventions on outcomes affecting patients and physicians, including objective measures when reported (PROSPERO ID: CRD42022309326).
Methods
Pubmed, Embase, and Cochrane Central Register of Controlled Trials were searched on January 13th, 2022. We included experimental or quasi-experimental studies that examined handoff communication between inpatient physicians and reported patient clinical, patient experiential, physician experiential, or cost and utilization outcomes. Studies were excluded if they examined handoffs between facilities or levels of care, or only reported subjective measures of patient safety or physician experience. Risk of bias was assessed using the ROBINS-1 and RoB-2 tools.
Results
Of the 42 included studies, six were randomized controlled trials. Most studies were conducted at academic centers (67%) and involved only residents (64%). An educational intervention was used in 52% of studies and a structural intervention was used in 43%, with 9% using both. Adverse events were significantly improved in three of 16 studies, medical errors in three of seven studies, and length of stay in three of seven studies. Four studies examined mortality, and none reported a significant improvement. Studies that used both structural and educational components reported significant improvements more frequently.
Conclusions
The literature is mixed on the impact of efforts to improve handoffs, though there are few randomized trials. Few studies reported patient experiential or cost/utilization outcomes, or involved hospitalist physicians, which represent potential areas for future research.
INTRODUCTION
With the introduction of duty hour restrictions in the United States in July 2003, transitions of care for hospitalized patients have become more frequent.1 During a transition of care, information is transmitted from one physician to another by a handoff, also referred to as a handover or a signout. Handoffs lacking in key information have been associated with adverse outcomes.2, 3 Types of handoffs include shift handoffs, weekend handoffs, and service handoffs, when one physician completes a block of time on the care team and another physician joins the care team. Over a 5-day hospitalization, an average patient will go through 15 handoffs.1 As a result, high-quality handoffs are critically important.
The Joint Commission established high-quality handoffs as a National Patient Safety Goal in 2006.4-6 The Joint Commission has outlined components of appropriate handoffs: patient summary, to-do list, contingency plans, code status, and opportunity for discussion. The American College of Surgeons and Association of Program Directors in Surgery include handoffs in their Surgery Resident Skills Curriculum.7 The Society of Hospital Medicine has also provided recommendations, including training new users, setting a dedicated time for handoffs, and prioritizing ill patients.5 Despite these recommendations, Internal Medicine residencies employ a wide range of strategies to implement and teach best practices in handoffs, which are not always consistent with national recommendations.8
In recognition of gaps in handoff quality, multiple interventions to improve handoffs have been evaluated with mixed evidence, including scheduling a time for handoff,9-12 setting best practices,13-15 role-playing,13, 16, 17 intrahospital advertising campaigns,11, 16, 18 and interventions with the electronic medical record (EMR).17, 19-21 Handoff mnemonics have been proposed to facilitate effective handoff, most notably including I-PASS (illness severity, patient summary, action list, situation awareness and contingency plans, and synthesis by receiver) and SBAR (situation, background, assessment, and recommendation). However, the evidence supporting all of these interventions has been criticized for relying on non-patient-relevant outcomes.22, 23
Several systematic reviews of handoff interventions have been conducted, most before 2015.5, 22-25 Since 2015, many studies have addressed handoffs, but published reviews have been narrow in scope, for example, focusing specifically on the SBAR handoff mnemonic,26 the impact of educational interventions,27 or the role of electronic handoffs.28 To inform efforts to improve practice, we set out to perform a current, broad, rigorous review of handoff interventions targeting physicians.
METHODS
Scope of review and search strategy
Literature searches were conducted on January 13th, 2022 in the following bibliographic databases: Pubmed, Embase (Embase.com) and Cochrane Central Register of Controlled Trials (Wiley). The search strategy had two components, generally related to clinical handoffs and physician/patient outcomes. Physician-related terms included those for physicians in general, as well as for hospitalists specifically. The search terms used were subject headings (MeSH, Emtree) and/or keywords in the title, abstract, and author fields of the database records. Boolean operators OR and AND were used to combine the search terms and the search strategy components. No limits were applied. The search results were de-duplicated in Covidence, which is a software program that facilitates systematic reviews.29
Inclusion and exclusion criteria
Studies were included if they were experimental or quasi-experimental evaluations of efforts to improve handoff communication between inpatient physicians at change of shift or change of service. Studies were excluded if they had no comparison group or relied on a historical cohort as a comparison group. Studies of handoffs between facilities or handoffs between levels of care were also excluded.
Outcomes of interest were categorized as patient clinical, patient experiential, physician experiential, and cost and utilization. We limited the review to studies that reported objective measures for these outcomes, excluding studies relying on physician subjective assessment of patient safety or user experience and those using physician satisfaction as an outcome.
Selection process
Each title and abstract was reviewed for inclusion independently by two reviewers (J. A. D., M. K., J. W., N. R., R. M., C. B., S. C., D. K.). All studies were reviewed using Covidence.29 Full-text review was performed independently by two of the same group of reviewers. Any disagreements were resolved by consensus or, if consensus was not reached, by a third reviewer.
Data collection
We collected data on study design, setting, number of hospitals involved, type of wards, and study duration. We recorded participant specialty and training level, handoff type (e.g., shift or service), handoff template format (if applicable), and whether the intervention included a structural change or an educational component. Specific outcomes of interest were also recorded.
Data from each study was extracted by two authors (J. A. D., M. K., J. W., N. R., R. M., C. B., S. C., D. K.) and recorded in AirTable, a cloud-based spreadsheet client. Descriptive statistics were calculated in Microsoft Excel. Given the heterogeneity of interventions and outcomes, no meta-analysis was planned or conducted.
Risk of bias assessment
For randomized trials, risk of bias was assessed using the RoB-2 (Risk of Bias) tool.30 For non-randomized trials, risk of bias was assessed using the ROBINS-1 (Risk Of Bias in Non-randomized Studies) tool.31
RESULTS
Study selection
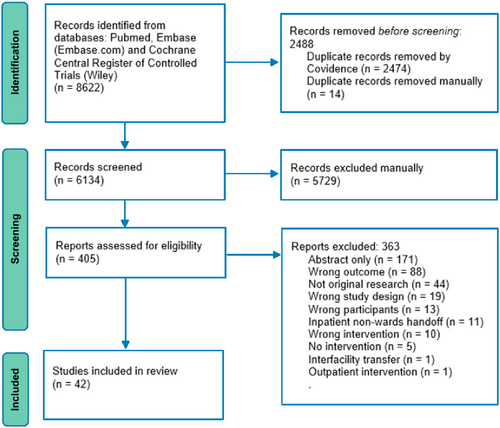
The initial literature search yielded 8662 records, from which 42 studies met the inclusion criteria (Figure 1).9-21, 32-60
Study characteristics
Of the 42 studies, six were randomized controlled trials.10, 11, 34, 35, 37, 38 The remaining 36 studies were observational, primarily preintervention/postintervention. Most studies were conducted at a single-center (n = 37, 90%), at academic centers (n = 28, 67%), and in the United States (n = 23, 55%). Most (n = 23, 55%) were published in 2015 or later, with three published before 2010.33, 35, 39
Studies were most often conducted in Medicine units (n = 17, 40%), followed by Surgery units (n = 14, 33%) and Pediatrics units (n = 4, 10%). Most studies (n = 27, 64%) involved only residents, while the other studies involved physicians from varying levels of training (n = 7, 17%), attending physicians only (n = 2, 5%), or did not specify (n = 6, 14%). The median study duration was 4.5 months (IQR 2.25–10). The most common type of handoffs studied were shift handoffs (n = 32, 76%), followed by weekend handoffs (n = 9, 21%) and service handoffs (n = 1, 2%). Full study characteristics are shown in Table 1.
| Author and year | Country | Setting | Study design | Participants | Type of handoff | Interventions | Outcomes and results |
|---|---|---|---|---|---|---|---|
| Airan-Javia 2012 | United States | Academic center (multi-hospital) | Pre/post | Internal Medicine residents | Shift | Didactic lecture content | Recall of code-related communication failures: 22.3% pre vs. 4% post, p = 0.001 Percentage of communication failures related to overnight tasks: 8.8 pre vs. 2.4 post, p = 0.03 |
| Akhunbay-Fudge 2014 | United Kingdom | Community teaching (single hospital) | Pre/post | Internal Medicine residents | Weekend | Introduced new “proforma” | Difficulty understanding patient notes (on a 10-pt scale): 5.7 pre vs. 0.8 post, no p-value reported Time to find ceiling of care (sec): 153 pre vs. 5 post, no p-value reported |
| Alolayan 2017 | Saudi Arabia | Academic center (single hospital) | Pre/post | Oncologists | Shift | Outside of EMR electronic intervention, Setting best practices/guidelines, Advertising campaign, Email reminders | Length of stay: Reported in run chart; no p-value reported |
| Atkinson 2015 | United States | Academic center (single hospital) | Pre/post | Surgery residents, Surgery fellows | Shift | Within EMR electronic intervention | Handoff associated adverse event: 1 pre vs. 0 post, no p-value reported Satisfaction score: 7.5 pre vs. 7.5 post, no p-value reported Frequency of AM information not communicated: 3.2 pre vs. 2 post, no p-value reported |
| Clanton 2017 | United States | Academic center (single hospital) | Randomized trial | Surgery residents | Shift | Standard scheduled face-to-face handoff, Setting best practices/guidelines, Role-playing/simulation, “Training and education” in addition to simulation. | Mortality (per 100 admissions): 4.3 focused vs. 3.8 formal, p = 0.392 Any adverse event (per 100 admissions): 17.5 focused vs. 15.9 formal, p = 0.118 Any negative event: 23.6 focused vs. 22.9 control, p = 0.556 ICU LOS (d): 6.53 focused vs. 5.92 formal, p = 0.727 LOS (d): 5.88 focused vs. 5.50 formal, p = 0.024 Duration of handoff (min): 6.7 focused vs. 20.6 formal, p < 0.001 |
| Culwick 2014 | United Kingdom | Community teaching (single hospital) | Pre/post | Surgery residents | Weekend | Outside of EMR electronic intervention | Satisfaction with handover system: 7.1% pre vs. 85.7% post, no p-value reported Average time to locate a patient (sec): 21 pre vs. 4 post, no p-value reported |
| Dean 2018 | United Kingdom | Academic center (single hospital) | Pre/post | Surgery residents | Weekend | Outside of EMR electronic intervention, Residents asked to use a standardized paper handoff document, and patient-level data tracked in EMR | LOS: 10.21 pre vs. 8.67 days post, p = 0.0356 Weekend discharges: 39.07 pre vs. 48.93 post, p = 0.0034 Cost savings associated with reduced LOS (Not reported as a formal outcome, however the authors do extrapolate based on reduced LOS) |
| Din 2012 | United Kingdom | Academic center (single hospital) | Pre/post | Surgery junior doctors, registrars, and nurses | Weekend | Outside of EMR electronic intervention, Written reference materials (verbal, email, and poster communication) | Discharge over weekend: 5% pre vs. 20% post, no p-value reported Average hours spent on ward rounds: 3.5 pre vs. 3 post, no p-value reported Confidence in handoffs: 65% pre vs. 78% post, no p-value reported |
| Fryman 2017 | United States | Academic center (single hospital) | Several PDSA cycles | Internal Medicine residents | Shift | Didactic lecture content | Adverse events: χ2 4.8, p = 0.04 Events that should have been anticipated or discussed during handoff but were not: χ2 9.6, p = 0.003 |
| Gagnier 2016 | United States | Academic center (single hospital) | Pre/post | Surgery residents | Shift | Significant educational component in the development of the handoff. Also notes that residents were “briefed” |
Any adverse events: 59.7% pre vs. 51.7% post Number of events per patient: 1.02 pre vs. 0.75 post, no p-value reported Hospital stay in mean days: 3.33 pre vs. 2.85 post Incidence risk ratio of control vs. test for adverse event in adjusted modified Poisson regression: 0.727, p = 0.044 Time to complete handoff: 3 doctors/60%, 1/20%, vs. 9 doctors 60%/2 doctors 13% in the 10 min vs. 30 min categories, no p-value reported |
| Gardezi 2014 | United Kingdom | Community teaching (single hospital) | Pre/post | Internal Medicine residents | Weekend | Implementation of checklist for handoff | Number of pages over the weekend for elements of the checklist: 83 pre vs. 32 post, no p-value reported |
| Gibbons 2016 | Ireland | Academic center (single hospital) | Several PDSA cycles | Surgery faculty and residents | Weekend, Other | Didactic lecture content | Weekend discharges: 10.6% pre vs. 14.8% post, p < 0.05 Length of stay: 13.0 days pre vs. 5.4 days post, p < 0.05 Emergency response team calls: 7 pre vs. 4 post, no p-value reported Re-admissions: 0 pre vs. 0 post, no p-value reported |
| Goldraij 2021 | Argentina | Community nonteaching (single hospital) | Pre/post | Palliative care faculty | Shift | Didactic lecture content | Percentage of families reporting patient comfort over night: 65% pre vs. 87% post, no p-value reported |
| Graham 2013 | United States | Academic center (single hospital) | Pre/post | Internal Medicine residents | Shift | Within EMR electronic intervention, Standard scheduled face-to-face handoff | Adverse events: IRR 0 (0, 3.11), p = 0.41 Near misses: IRR 0 (0, 1.04), p = 0.056 |
| Huth 2016 | Canada | Academic center (single hospital) | Pre/post | Pediatrics residents | Shift | Setting best practices/guidelines, Role-playing/simulation, Didactic lecture content, Written reference materials, Advertising campaign | Handoff duration: Decreased by 1.7 min (p = 0.38) |
| Kenny 2014 | United Kingdom | Community teaching (single hospital) | Pre/post | Surgery residents | Shift | Outside of EMR electronic intervention | Overall satisfaction: 0% pre vs. 100% post, no p-value reported Daily time updating the list (min): 95 pre vs. 53 post, no p-value reported |
| Krowl 2018 | United States | Academic center (single hospital) | Pre/post | Internal Medicine residents | Shift | Didactic lecture content, small group learning | Number of rapid responses initiated per day: 1.05 pre vs.0.98 post, p = 0.345 |
| Lee 1996 | United States | Academic center (single hospital) | Randomized trial | Internal Medicine residents | Shift | Standardization of handoff card | LOS (days): 4.6 control to 5 intervention, no p-value reported Poor sign out (assessed nightly): 14.9% control vs. 5.8% intervention, p = 0.016 |
| Lo 2016 | United States | Teaching (single hospital) | Pre/post | Pediatric hospitalists | Shift | Within EMR electronic intervention, Email reminders, Written reference materials | Median total handoff time (min): 84 pre vs. 61 post, p < 0.001 |
| Mueller 2016 | United States | Academic center (single hospital) | Pre/post | Internal Medicine residents, Surgery residents | Shift, Service | Outside of EMR electronic intervention | Total Medical Errors: Decreased, IRR 0.49 (0.42–0.58), p < 0.001 Medical errors owing to mistakes in handoff (per 100 patient days): 2.47 pre vs. 0.95 post, p < 0.001 |
| Nabors 2015 | United States | Academic center (single hospital) | Pre/post | Internal Medicine residents | Shift | Within EMR electronic intervention, Setting best practices/guidelines | Duration of sign out (minutes): 25.5 pre vs. 22.7 post, p = 0.0338 |
| Patel 2014 | United Kingdom | Teaching (single hospital) | Pre/post | Psychiatry residents | Weekend | Within EMR electronic intervention | Mortality rate: 16.5% pre vs. 12.5% post, no p-value reported Percentage of deaths during weekend (OR): 0.37, p = 0.07 |
| Payne 2012 | United States | Academic center (single hospital) | Non-randomized parallel | Internal Medicine residents | Shift | Outside of EMR electronic intervention | Near-miss events as perceived by the physician: 55% control vs. 31.5% intervention, p = .0341 |
| Pennell 2017 | United Kingdom | Teaching (single hospital) | Pre/post | Multidisciplinary | Shift | Standard scheduled face-to-face handoff, Setting best practices/guidelines, Multidisciplinary handoff | Handoff Duration (minutes): 17 pre vs. 23 post, p = 0.041 |
| Petersen 1998 | United States | Academic center (single hospital) | Pre/post | Internal Medicine residents | Shift | Outside of EMR electronic intervention | Preventable adverse event: 1.71% pre vs. 1.23% post, no p-value reported Preventable adverse events during cross coverage: 6 pre vs. 9 post, p > 0.10 |
| Piscioneri 2011 | Australia | Teaching (single hospital) | Pre/post | Surgery faculty and residents | Shift, Weekend | Standard scheduled face-to-face handoff | Completion rate of tertiary trauma survey: 29.6% pre vs. 86.1% post, no p-value reported Time from admission to tertiary survey (hours): 30.6 pre vs. 32.8 post, no p-value reported |
| Rao 2012 | Australia | Academic center (single hospital) | Pre/post | Internal Medicine residents | Shift | Outside of EMR electronic intervention, setting best practices/handoffs | Weekend discharges: 14.2% pre vs. 20.39% post, p < 0.001 Emergency medical calls: 7.47% pre vs. 5.49 post, p = 0.01 |
| Raval 2015 | United States | Academic center (single hospital) | Pre/post | Surgery faculty and residents | Shift | Moved from Microsoft access directory to moving handoff into Epic. | Codes outside of ICU: 2 pre vs. 3 post, p = 0.65 Serious safety events: 3 pre vs. 0 post, p = 0.08 Adverse drug event related to abx prescribing: 3 pre vs. 1 post, p = 0.32 Readmission rate: 3.0% pre vs. 2.9% post, p = 0.85 GI surgery infection rate: 6.3% pre versus 4.0% post, p ≤ 0.01 Average time spend on list maintenance per person per week in min.: 155.75 pre vs. 112.59 post, p = 0.16 |
| Sadiq 2021 | United Kingdom | Academic center (single hospital) | Pre/post | Surgery faculty and residents | Shift | Advertising campaign | Length of trauma handover (in minutes): 19.5 pre vs. 23.8 post, p = 0.26 Length of admissions handover (in minutes): 19.7 vs. 24.3, p = 0.28 |
| Salerno 2009 | United States | Teaching (single hospital) | Pre/post | Psychiatry, Family Medicine, and Transitional Year interns | Shift | Didactic lecture content | Sign out accuracy (day to night): 82% vs. 87%, p = 0.52 Missed issues requiring overnight management: 90% pre vs. 60% post, p = 0.03 |
| Schouten 2015 | United States | Academic center (single hospital) | Retrospective chart review | Adult hospitalists | Shift | No intervention | Rapid response team call: 4/305 control vs. 5/500 intervention, p = 0.68 Code team calls: 0/305 control vs. 1/500 intervention, p = 0.43 Transfer to higher level of care: 2% control vs. 2% intervention, p = 0.93 30-day readmission: 16% control vs. 13% intervention, p = 0.23 Hospital LOS (median hours): 66.5 control vs. 70.3 intervention, p = 0.30 Adverse events: Temporary harm with intervention: 4 control vs. 7 intervention, p = 0.92 Adverse events: Temporary harm with prolonged hospitalization: 7 control vs. 8 intervention, p = 0.53 Adverse events: Permanent harm: 0 control vs. 1 intervention, p = 0.44 Adverse events: Intervention to sustain life: 0 control vs. 6 intervention, p = 0.14 Adverse events: Death: 0 control vs. 0 intervention, p = 1.00 Total adverse events per 100 admissions: 3.61 control vs. 4.40 intervention, p = .59% admissions with adverse event: 2.6% control vs. 3.2% intervention, p = 0.64 |
| Singh 2019 | United States | Academic center (single hospital) | Pre/post | Internal Medicine residents | Shift | Setting best practices/handoffs | Mean daily rate of nonlethal adverse events: 0.54 pre vs. 0.28 post, p = 0.10 |
| Skelton 2019 | United Kingdom | Teaching (multi-hospital) | Pre/post | Psychiatry trainees and general practice trainees | Weekend | Written reference materials, Prerecorded content | Adverse incidents: 21 pre vs. 12 post, p = 0.29 Whether handover improved patient care: 100%, no p-value reported Whether information during handover was inadequate before intervention: 96%, no p-value reported |
| Sonoda 2021 | United States | Academic center (single hospital) | Pre/post | Family Medicine residents | Shift | Didactic lecture content, small group learning | Unexpected floor calls: 54.4% pre vs. 36.2% post, p < 0.05 Overall medical errors: 6 pre vs. 10 post, no p-value reported |
| Starmer 2013 | United States | Academic center (single hospital) | Pre/post | Pediatrics residents | Shift | Setting best practices/guidelines, Within EMR electronic intervention, Outside of EMR electronic intervention, Standard scheduled face-to-face handoff, Didactic lecture content | Medical errors per 100 admissions: 33.8 pre vs. 18.3 post, p < 0.001 Preventable adverse events per 100 admissions: 3.3 pre vs. 1.5 post, p = 0.04 Nonintercepted potential adverse events: 7.3 pre vs. 3.3 post per 100 admissions (p = 0.002) Intercepted potential adverse event: 5.0 pre vs. 8.3 post per 100 admissions, p < 0.001 Errors with little or no potential for harm: 8.3 pre vs. 5.2 post per 100 admissions, p = 0.04 Nonpreventable adverse events: 1.7 pre vs. 1.6 post per 100 admissions, p = 0.91 Time spent with patients (percentage of time in 24 h period at bedside): 8.3 pre vs. 10.6 post, p = 0.03 Time spent at the computer: 24.2% pre vs. 23.2% post, p = 0.64 |
| Starmer 2014 | US and Canada | Academic center (multi-hospital) | Pre/post | Pediatrics residents | Shift | Within EMR electronic intervention, Role-playing/simulation, Prerecorded content, small group learning | Medical Errors and Adverse Events: 24.5 pre vs. 18.8 per 100 admissions post, p < 0.001 Preventable adverse events: 261 pre vs. 173 post, p < 0.001 Duration of oral handoffs (minutes per pt): 2.4 pre vs. 2.5 post, p = 0.55 Percent of time spent w pts/families: 11.8% pre vs. 12.5% post, p = 0.41 Time spent writing/editing handoffs (in 24 h): 0.6% and 1.3%, p = 0.54 |
| Tam 2018 | Canada | Academic center (single hospital) | Randomized trial | Internal Medicine residents | Shift | Standard scheduled face-to-face handoff, Didactic lecture content | All medical errors: RR 1.18 [0.49,2.28], p = 0.72 Proportion of patients transferred to ICU (RR): 1.20 [0.44,3.32], p = 0.72 Proportion of patients evaluated by critical care (RR): 0.89 [0.54,1.48], p = 0.66 In-hospital mortality rate (RR): 0.93 [0.63,1.39], p = 0.73 Preventable adverse events (RR): 0.52 [0.09,3.10], p = 0.47 Proportion of patients receiving resource utilization overnight (RR): 1.50 [1.12,2.00], p = 0.01 |
| Telem 2011 | United States | Academic center (single hospital) | Pre/post | Surgery residents | Shift | Teaching sessions, pre-recorded content, role-playing/simulation | Sentinel events - major morbidity/mortality errors: 1 overall, not significant Percentage of erroneous orders in order entry: 14.5 pre vs. 12.2 post, p = 0.003 |
| VanEaton 2010 | United States | Academic center, Community teaching (multi-hospital) | Randomized trial | Internal Medicine residents, Surgery residents | Shift | Within EMR electronic intervention | Medical errors per 1000 patient-days: 6.33 control vs. 5.61 intervention, p = 0.68 Adverse drug events (OR): 1.10 [0.69, 1.74], p = 0.70 Resident-reported events per team: 6.0 control vs. 6.6 intervention, p = 0.66 |
| Wohlauer 2012 | United States | Academic center (single hospital) | Pre/post | Internal Medicine residents, Surgery residents | Shift | Within EMR electronic intervention | Mean time pre-rounding (min): 62.7 pre vs. 51.9 post, p = 0.0064 Missed patients on rounds-less than once per month: 57% pre vs. 70% post, p = 0.0037 |
| Wolfe 2018 | United States | Academic center (single hospital) | Pre/post | Surgery faculty and residents | Shift | Standard scheduled face-to-face handoff | Length of stay: 6 days pre vs. 4.9 days post, p < 0.0001 Intensive care length of stay: 2.1 days pre vs. 1.5 post, p < 0.0001 Total vent days: 1.3 pre vs. 0.9 post, p = <0.0001 |
| Wolinska 2021 | Canada | Academic center | Pre/post | Pediatric surgeons | Shift | Didactic lecture content, Ongoing coaching | Length of handoff in minutes: 20 pre vs. 25 post, p < 0.01 |
Study interventions
All but one study used a shared handoff template as part of the intervention, most often an electronic template (Figure 2). The components of the handoff template were described in detail in 37 studies (88%): 6 (14%) used I-PASS, 6 (14%) used SBAR, and the remainder created a novel handoff template.
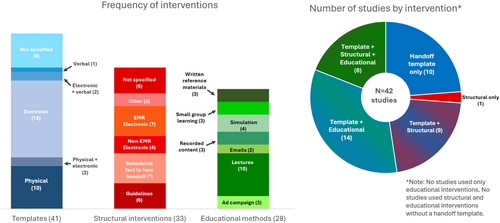
About half of the studies (n = 22, 52%) included an educational intervention, most often lectures (n = 10, 45%), with 10 (45%) using more than one educational approach (Figure 2). Most educational interventions were time-limited, including a single educational session in eight studies (36%) and multiple sessions in seven (31%). Only four (18%) involved ongoing educational series.
A structural (i.e., systems improvement) intervention was reported in 24 (57%) studies, among which 6 (25%) included multiple structural interventions. The most common structural interventions were scheduling a standard face-to-face handoff (n = 7, 39%), electronic interventions within the EMR (n = 7, 39%), and setting best practices/guidelines (n = 6, 33%). Electronic interventions within the EMR included an overnight event logger19 and a prerounding tool that automatically incorporated relevant data.20
Intervention types were similar across studies involving different specialties and handoff types. Of the studies with internal medicine physicians, 6 of 19 used educational interventions (32%) and 6 of 19 used structural interventions (32%). Of the studies with surgeons, 8 of 17 used educational interventions (47%) and 6 of 17 used structural interventions (35%). Of the 34 studies examining shift handoff, 17 (50%) used educational interventions and 12 (35%) used structural interventions. Of the 9 studies examining weekend handoff, 4 (50%) used educational interventions and 2 (22%) used a structural intervention.
A greater proportion of studies with internal medicine physicians focused on weekend handoffs compared to the studies with surgeons. Of the 19 studies involving internal medicine physicians, 13 (68%) examined shift handoffs, and 6 (32%) examined weekend handoffs. Of the 17 studies involving surgeons, 16 (94%) examined shift handoffs and 1 (6%) examined weekend handoffs. The proportion of types of handoffs studied were similar across studies involving different levels of training. Of the 7 studies involving faculty (5 of which also involved trainees), 6 (86%) examined shift handoffs and 2 (29%) examined weekend handoffs. Of the 30 studies involving trainees, 25 (83%) examined shift handoffs and 5 (17%) examined weekend handoffs.
Study outcomes
The most common outcomes were patient clinical (n = 28, 67%) and physician experiential outcomes (n = 25, 60%) (Figure 3 and Table 2). Few studies measured patient experiential (n = 1, 2%) or cost/utilization outcomes (n = 2, 5%). Overall, five (83%) of the RCTs and 17 (47%) of observational studies found improvement with the handoff intervention. The most commonly reported clinical outcome was rate of adverse events (AE), which was included in 16 studies.10, 11, 15-17, 21, 32, 34, 37, 39, 51, 53, 55-57, 60 Three studies found improved rates of AEs, including in preventable AEs in the one study that reported this outcome. These three studies were all observational; two used a combined intervention of a handoff template, structural intervention, and educational intervention.17, 56, 57 Seven studies reported rates of medical errors,10, 16, 17, 32, 34, 54, 56 three reported significant improvement.17, 32, 56 Two of these three studies used a combination of handoff template, structural intervention, and educational intervention, while the third study used a structural intervention only. No studies that evaluated mortality (n = 4), number of codes (n = 2), or number of ICU transfers (n = 1) found improvements with handoff interventions. Seven studies reported length of stay,11, 14, 35, 41, 52, 55, 58 and three reported significant improvement.11, 52, 58
| Study author, year | Mortality | Adverse events | Length of stay | Handoff duration |
|---|---|---|---|---|
| Alolayan 2017 | Reported in run chart; no p-value reported | |||
| Atkinson 2015 | Handoff associated adverse event: 1 pre vs. 0 post, no p-value reported | |||
| Clanton 2017 | Mortality (per 100 admissions): 4.3 focused vs. 3.8 formal, p = .392 | Any adverse event (per 100 admissions): 17.5 focused vs. 15.9 formal, p = 0.118 | LOS (days): 5.88 focused vs. 5.50 formal, p = 0.024 | Duration of handoff (min): 6.7 focused vs. 20.6 formal, p < 0.001 |
| Dean 2018 | LOS (days): 10.21 pre vs. 8.67 days post, p = 0.0356 | |||
| Fryman 2017 | Adverse events: χ2 4.8, p = 0.04 | |||
| Gagnier 2016 | Any adverse events: 59.7% pre versus 51.7% post | Hospital stay in mean days: 3.33 pre vs. 2.85 post | ||
| Gibbons 2016 | Length of stay: 13.0 days pre vs. 5.4 days post, p < 0.05 | |||
| Graham 2013 | Adverse events: IRR 0 (0, 3.11), p = 0.41 | |||
| Huth 2016 | Handoff duration: Decreased by 1.7 min (p = 0.38) | |||
| Lee 1996 | LOS (days): 4.6 control vs. 5 intervention, no p-value reported | |||
| Lo 2016 | Median total handoff time (min): 84 pre vs. 61 post, p < 0.001 | |||
| Mueller 2016 | Total Medical Errors: Decreased, IRR 0.49 (0.42–0.58), p < 0.001 | |||
| Nabors 2015 | Duration of sign out (minutes): 25.5 min pre vs. 22.7 post, p = 0.0338 | |||
| Patel 2014 | Mortality rate: 16.5% pre vs. 12.5% post, no p-value reported | |||
| Pennell 2017 | Handoff Duration (minutes): 17 pre vs. 23 post, p = 0.041 | |||
| Petersen 1998 | Preventable adverse event: 1.71% pre vs. 1.23% post, no p-value reported | |||
| Raval 2015 | Serious safety events: 3 pre vs. 0 post, p = 0.08 | |||
| Schouten 2015 | Death: 0 control vs. 0 intervention, p = 1.00 | Total adverse events per 100 admissions: 3.61 control vs. 4.40 intervention, p = .59% admissions with adverse event: 2.6% control vs. 3.2% intervention, p = 0.64 | Hospital LOS (median hours): 66.5 control versus 70.3 intervention, p = 0.30 | |
| Singh 2019 | Mean daily rate of nonlethal adverse events: 0.54 pre vs. 0.28 post, p = 0.10 | |||
| Skelton 2019 | Adverse incidents: 21 pre vS. 12 post, p = 0.29 | |||
| Starmer 2013 | Preventable adverse events per 100 admissions: 3.3 pre vs. 1.5 post (p = 0.04) | |||
| Starmer 2014 | Preventable adverse events: 261 pre vs. 173 post, p < 0.001 | Duration of oral handoffs (minutes per pt): 2.4 pre versus 2.5 post, p = 0.55 | ||
| Tam 2018 | In-hospital mortality rate (RR): 0.93 [0.63,1.39], p = 0.73 | Preventable adverse events (RR): 0.52 [0.09,3.10], p = 0.47 | ||
| Telem 2011 | Sentinel events - major morbidity/mortality errors: 1 overall, not significant | |||
| VanEaton 2010 | Adverse drug events (OR): 1.1, p = 0.7 | |||
| Wolfe 2018 | Length of stay: 6 days pre vs. 4.9 days post, p < 0.0001 | |||
| Wolinska 2021 | Length of handoff in minutes: 20 pre vs. 25 post, p < 0.01 |
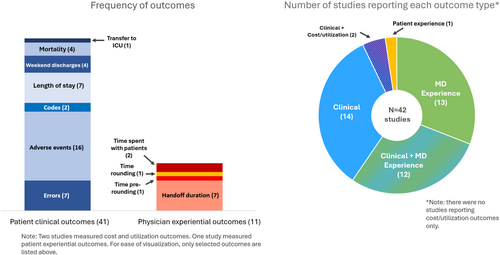
Physician experiential outcomes were diverse but most often related to time saved. The most common time-related outcome was handoff duration, which was reported in seven studies,9, 11, 13, 17-19, 42 two of which noted significant improvement. Of the two significant studies, one instituted a handoff template, along with ongoing education.42 The second study instituted a less-formal handoff structure and noted significant decrease in the length of handoffs, without any change in mortality or adverse events.11 Time spent rounding or pre-rounding did not change significantly in the two studies reporting those outcomes.20, 50 Two studies reported time spent with patients or families,17, 56 with one showing a significant improvement after implementation of a new handoff template, accompanying by ongoing education.56 Other outcomes included missed issues requiring overnight management,33 number of erroneous orders,16 and number of weekend pages.48
The single study measuring a patient experiential outcome found a significant increase in the percentage of families reporting patient comfort overnight after a handoff template was instituted along with an educational intervention.49 Two studies reported outcomes related to cost and utilization. One projected cost savings based on reduced length of stay after a standard handoff template was instituted,41 and, in the second, the intervention (a standardized template, scheduled time for handoff, and educational series) was associated with a significant increase in overnight health resource utilization.10
Risk of bias assessment
Most non-randomized studies (75%) had a serious risk of confounding bias, although the risk of selection bias and bias due to classification of interventions was generally low (Supporting Information S1: Figure 1 and Table 3). Other biases were mixed (Supporting Information S1: Figure 1).
Among the six randomized trials, risk of bias was mixed for risk of bias (RoB) due to the randomization process, missing outcome data, and outcome measurement and low for RoB due to deviations from the intended intervention and selection of reported results. See Supporting Information S1: Figure 1 and Table 4 for details.
DISCUSSION
This review provides an updated view of the literature on handoff interventions, with over half of the studies published after 2014. Although several reviews have examined the effects of handoff interventions, most were conducted before 2015,5, 22-25 with more recent reviews taking a narrower scope than this review.27, 28, 32 We examined the effect of handoff interventions on patient clinical, patient experiential, physician experiential, and cost/utilization outcomes. Notably, only one study measured patient experiential outcomes.49 Overall, fewer than half of the studies reported a significant improvement in outcomes. Adverse events significantly improved in three of 16 studies evaluating that outcome, medical errors in three of seven studies, and length of stay in three of seven studies. No study reported a significant improvement in mortality, number of codes, or number of ICU transfers, although few studies reported these outcomes. The most commonly reported physician experiential outcome, handoff duration, improved significantly in two of seven studies.
Characteristics of successful interventions
The most commonly reported patient clinical outcome was the rate of adverse events (AE), with 3 of 16 studies that evaluated AEs reporting improvement.17, 56, 57 Two of these three studies used a combination of structural and educational components,17, 56 whereas among 13 studies without significant improvement, only two used both structural and educational interventions. This may indicate an increased effect from the combination of structural and educational interventions. Multimodal interventions have been shown to have greater durability (vs. educational or structural interventions alone) in several reviews of quality improvement initiatives.61-63
One study reported that a multimodal handoff intervention was associated with a significant increase in health resource utilization overnight (e.g., receiving IV fluids, antibiotics, imaging, or blood tests).10 However, it is unclear whether this represents a shift in healthcare utilization from day shift to night shift, or an overall increase in healthcare utilization.
Limitations of the literature
There are many limitations in the evidence addressing handoff interventions. Many studies reported only physician satisfaction as an outcome; we excluded these studies. Among included studies, the quality of the evidence was limited by a predominance of non-randomized and observational studies. Studies were often short, lasting a median of 4.5 months. Important patient-centered outcomes were rarely reported, including mortality (n = 4 studies), number of codes (n = 2), and patient experience (n = 1). Only six studies reported formal power calculations.10, 17, 33, 34, 56, 57 An additional eight studies reported underpowering as a potential limitation, but did not report formal calculations.11, 15, 32, 39, 42, 43, 45, 55 Definitions and measurement of key outcomes varied across studies. For example, medical errors were sometimes identified by residents,32 by attending physicians,10 or by dedicated research nurses.17, 56
Few studies examined weekend handoffs (n = 9, 21%) or service handoffs (n = 1, 2%), making it unclear whether these studies used different interventions than studies of shift handoffs and obscuring the understanding of the most effective approaches. Few studies involved faculty (n = 7, 17%), limiting the ability to fully evaluate or compare handoff types in that group.
In the risk-of-bias assessment, most of the non-randomized trials had a serious risk of confounding bias. Often, no information was reported to enable risk of bias assessment due to deviations from the intended interventions, or risk of bias assessment related to missing data. Many studies did not report a pre-registered protocol, indicating at least a moderate risk of bias in the selection of reported results. In the randomized trials, allocation concealment was often not performed or described.
Limitations of the review
In addition to issues in the underlying evidence, there are limitations to our review. Although we conducted a thorough search of the academic literature, we did not conduct a search of gray literature, which could have led to missed studies. We did not specifically target residents or trainees in our search. However, we found many studies of resident handoffs and are confident we captured most of the literature on trainee handoffs. We did not record information on funding sources; doing so may have elucidated biases or provided context for future researchers about the resources required to implement the interventions described. Many interventions involved the EMR, but we did not collect information about which specific EMR was used. It may be that certain EMRs lend themselves to easier or more successful implementation of handoff interventions.
To our knowledge, this is the largest systematic review of handoff interventions to date, including more than double the number of studies compared to recent reviews.26-28, 64 Our review clarifies the need for additional research on how to design handoffs to optimize outcomes. Future research should include outcomes related to patient experience and total admission cost and utilization, which have rarely been studied, and all studies of handoff interventions should include metrics related to patient safety. No studies were powered to measure the impact of handoff interventions on patient mortality, although there was no significant change in patient mortality in the four studies reporting that outcome.10, 11, 46, 55 There is a need for larger studies of handoff interventions that are powered to detect differences in mortality. Future research should focus on patient-centered outcomes that are clinically important, measurable, and likely to be sensitive to handoff quality- such outcomes likely include escalations in care intensity, adverse events, inappropriate medication prescribing, other medical errors, and length of stay. We also recommend that future studies report power calculations, rates of missing data, and use pre-registered protocols.
Importantly, only two studies examined handoffs by hospitalist physicians.40, 55 There has been substantial growth in the practice of hospital medicine in the last decade.65 As hospitalists are increasingly involved in inpatient handoffs, and have the ability to provide consistency for trainees, they should be a focus of future handoff research.
CONCLUSION
This broad systematic review of the impact of inpatient handoff interventions demonstrates the inconsistent effect of such interventions on studied patient and physician outcomes. For most outcomes, fewer than half of studies reported a significant improvement, including in adverse events, medical errors, mortality, length of stay, and handoff duration. Interventions combining educational and structural components may be more effective. The body of evidence was limited by many studies of short duration and lack of randomized trials. Given the critical importance of effective handoffs in ensuring patient safety, future studies should rigorously address multimodal interventions with measurement of important patient-centric outcomes.
The review was registered with PROSPERO, which is a database of prospectively registered systematic reviews (registration number: CRD42022309326). The review protocol can be accessed through PROSPERO.66
ACKNOWLEDGMENTS
Dr. Korenstein and Dr. Allen-Dicker conceptualized and designed the study. All authors performed abstract review, full-text review, and data abstraction. Dr. Kerwin analyzed the data. Dr. Kerwin drafted the manuscript and all authors contributed to editing and finalizing the manuscript. The work of Drs. Allen-Dicker, Wallins, Rao, Mara, Chimonas and Korenstein and Ms. Chilov supported in part by a Cancer Center Support Grant from the National Cancer Institute to Memorial Sloan Kettering Cancer Center (award number P30 CA008748).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.